The Science
Behind World-class Technology is Visionary Scientific Theory & Research
WHAT WE HAVE CONCRETE KNOWLEDGE OF…
What We Know
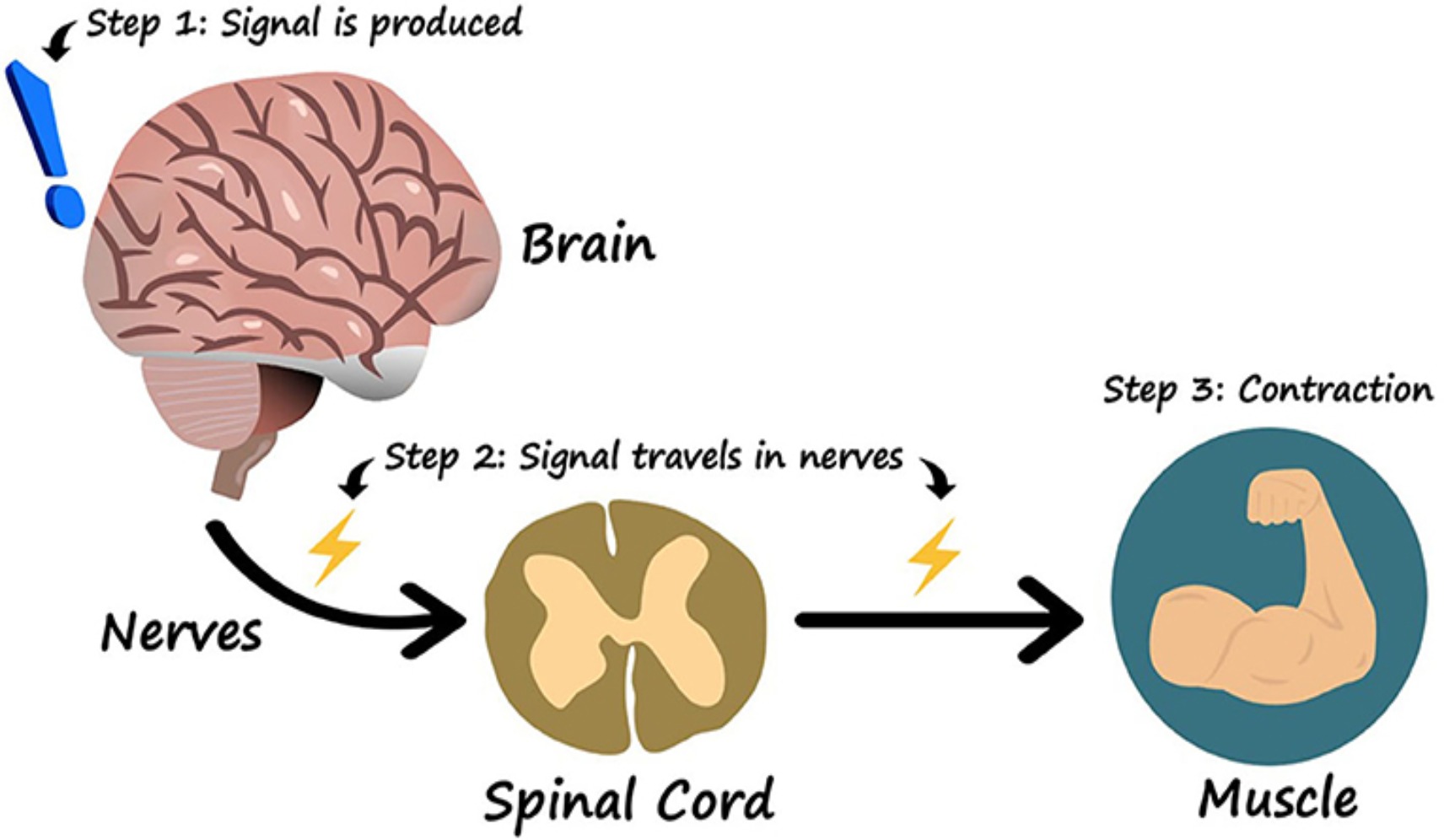
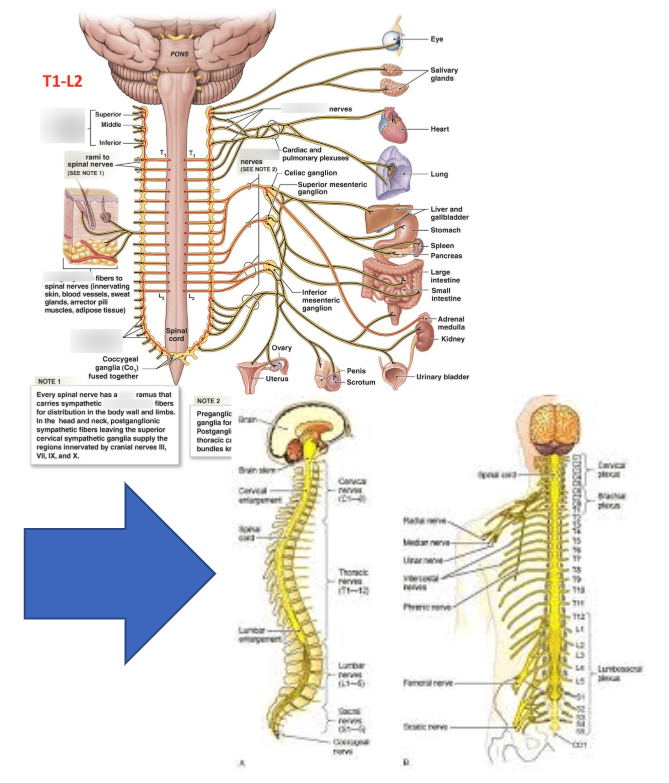
The traditional pathway of movement and communication is from the brain to the body.
This is referred to as the: Neuro-Muscle Axis
TO WHAT WE HAVE KNOWN FOR DECADES…
What We Know
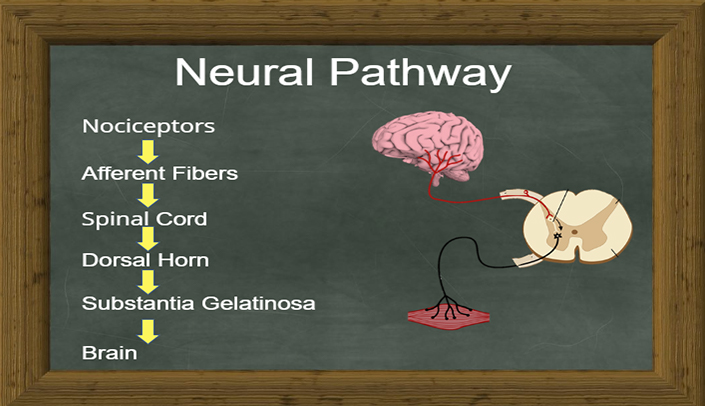
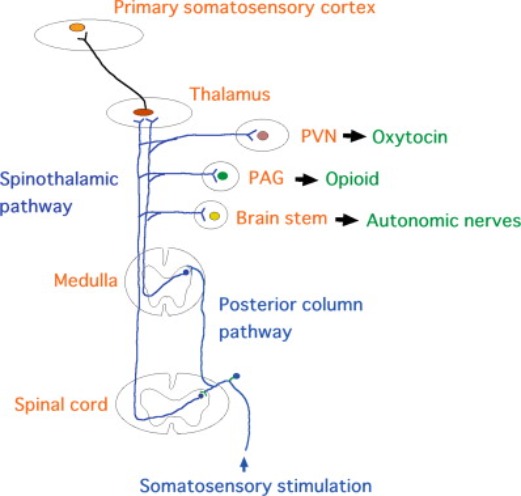
To date, what we understand about the “Body to Brain” axis is around the concept of being able to modulate pain via preferential attention to A-Alpha / A-Beta nerve fiber activation vs. A-Delta / C-Fiber pain stimulus.
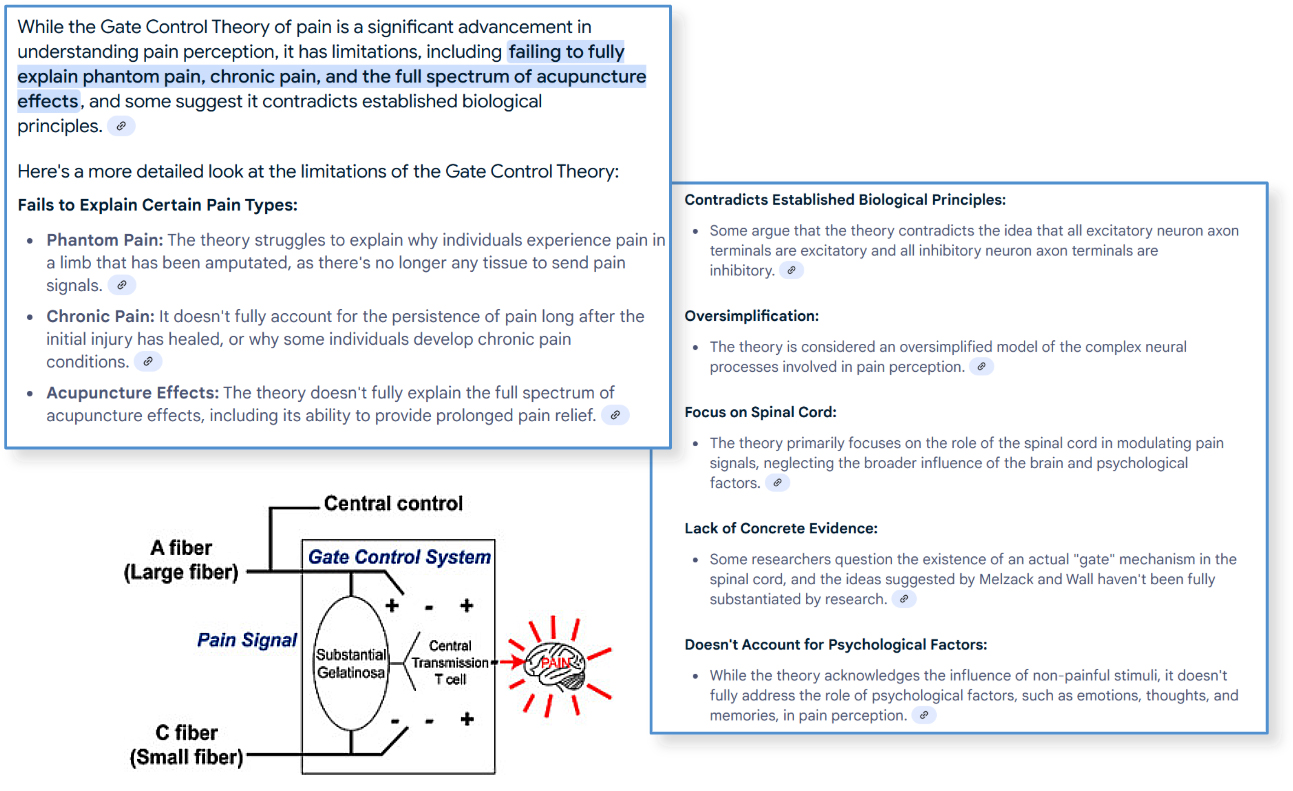
This is referred to as: GATE Theory
TO THE SCIENCE LIMITATIONS OF OUR TECHNOLOGIES…
Limitations
Since 1965, the collective medical community has not evolved beyond the concept that there are “gates” for communication afferent to the brain.
Is the science limited OR has the right delivery mechanism be invented yet?
TO WHAT WE ARE DISCOVERING…
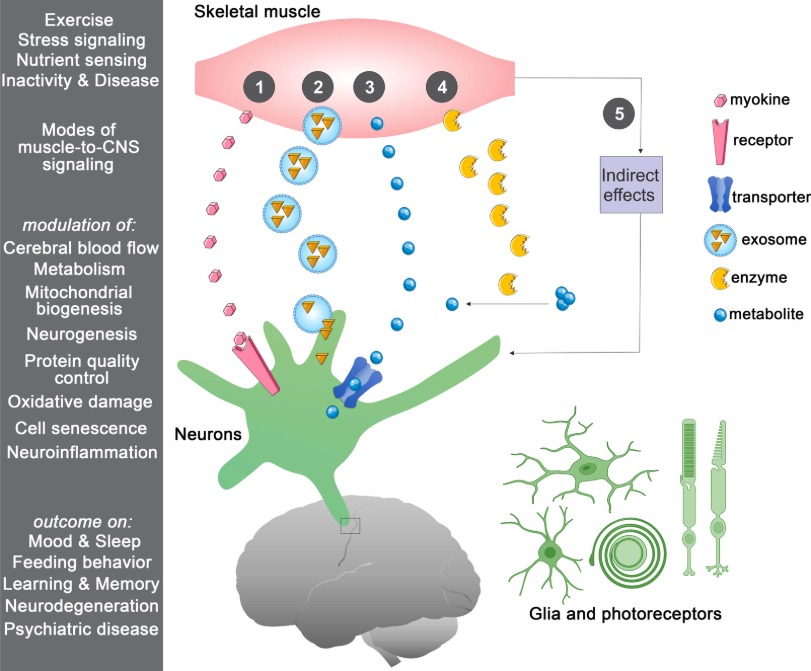
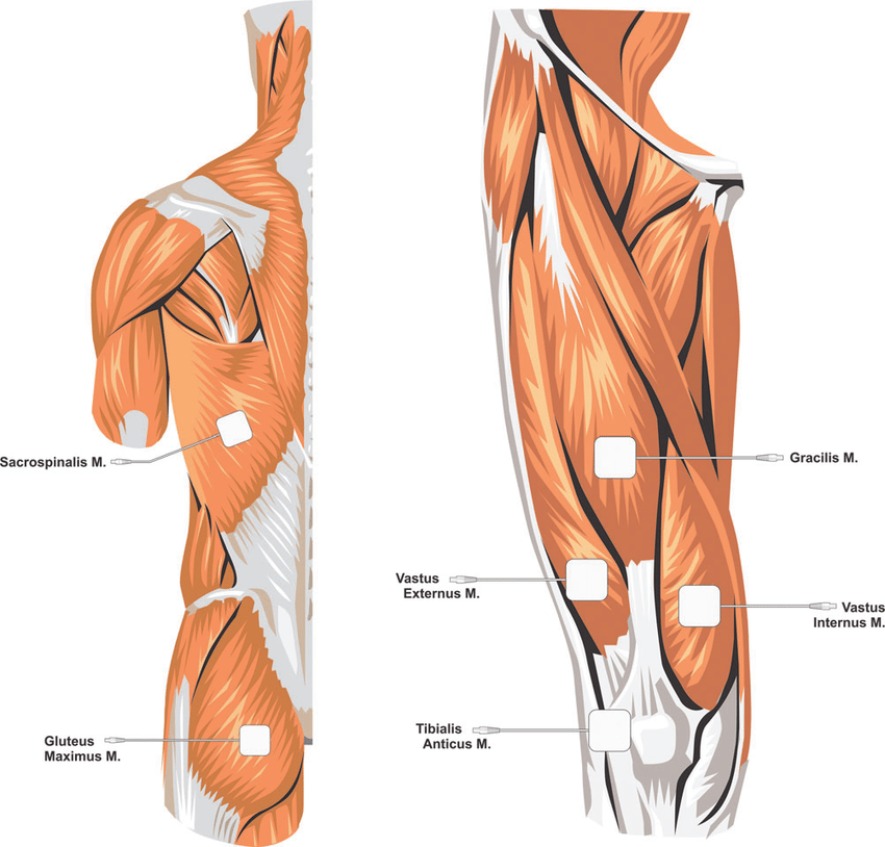
Because we are able to stimulate muscles locally and because recent research demonstrates that there is the ability for muscles to have an effect the brain afferently through myokines, then instead of there being just a Neuro-Muscle Axis….
We must refer to this new area of science as the: Muscle-Neuro Axis

WE NOW CAN ASK THE QUESTION…
What if the “mass” electrical signaling of Neuro20, delivered externally, taps into ALL THE GATES….
….thereby breaking the “negative cycles” among the Brain, Spinal Cord, and Body?…
Then Neuro20 is the first non-invasive, drug-free, Electrophysiological Neuromodulation to stimulate HORMESIS
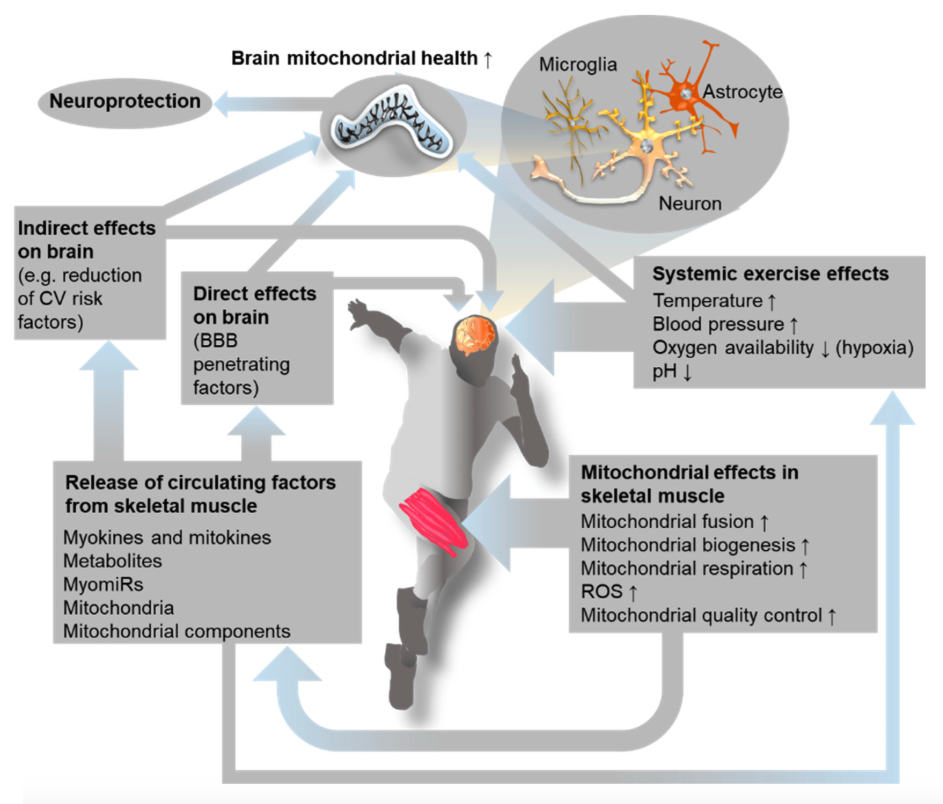
Hormesis: A biological phenomenon in which exposure to low doses of a substance or stressor that is typically harmful at higher doses can produce beneficial effects.
BECAUSE WE SEE THIS EFFECT VIA OTHER SIGNALING…
We Have Explored Other “Hormesis Mimicks” for Many Years
Other treatments that focus on “mass” signaling between the body and brain have shown benefits for overall health.
- Cryotherapy (Cold – Sensory)
- Sauna (Heat – Sensory)
- Intermittent Fasting (Digestive – Metabolic)
- Vagus Stimulator (CN X – Parasympathetic)
- Massage (Mechanical – Sensory)
- Exercise (Muscle Activation + Mechanical + Sensory + Multi-System Communication)
So, what if we apply safe mass electrical signaling?
AND IF EVERYTHING IS INTERCONNECTED…
We have identified that controlling a single “gate” can have positive impact on a signal pathway. We know there are benefits to “mass” signaling with other forms of treatment/therapy, and identified that muscle contractions can have an endocrine effect systemically that positively impacts the brain and body.
So as postulation, if a person is suffering from Mass Internal Dysfunctional Physiologic Signaling (MIDPS), then wouldn’t it be possible to reverse engineer this functional physiological signaling externally?
WE MUST LOOK TO OUR PAST…
For thousands of years humans have watched in awe how birds fly… We could not fly until the “Wright” technological advancements were invented. The underlying scientific principles were alway there, we just needed the right delivery system to take to the skies.

AS NEURO20 CARVES THE PATH TO MEDICAL DISCOVERY
”Mass External Electro-Physiological Signaling” (MEEPS) Definition: The application of mass peripheral electrical stimulation to generate systemic physiological signaling creating a positive cascading effect on movement, internal homeostasis, and overall function improving quality of life.
–Dr. Keith J. Cronin, DPT (Cronilectric Theory)